Quantification of Endogenous DMT Production During Extended Periods of Darkness
Andrew R. Gallimore, PhD., Noonautics.
DMT is established as an endogenous product of mammalian biochemistry, with concentrations in rat brain comparable to that of the neuromodulators serotonin and dopamine.¹ It has also been detected in the blood, urine, and cerebrospinal fluid of humans since the 1950s but, despite numerous attempts to link endogenous DMT production with the positive symptoms of schizophrenia, no definitive relationship has been found and its function and mechanism of regulation in humans remain unknown.²

Despite these gaps in our understanding, extended periods of darkness lasting several days to several weeks (“darkness retreats”) are known to induce highly visionary states that have been described anecdotally as indistinguishable from those induced by DMT. Since the production of the neurohormone melatonin, which is both chemically and biosynthetically closely related to DMT, is regulated by light exposure, it’s not entirely implausible that endogenous DMT production might also be induced during extended periods of darkness through an as yet unidentified regulatory mechanism.³ This hypothesis, however, has never been tested.
Since DMT can be detected in blood and urine, elevated endogenous DMT production ought to be detectable by quantifying the level of DMT in blood/urine samples acquired at intervals during an extended darkness retreat. Unfortunately, whilst blood sampling provides the most reliable measure of endogenous DMT production, performing venepuncture safely under conditions of complete darkness presents an obvious challenge. However, the repeated sampling from blood drawn from a canula inserted at the beginning of the retreat is a feasible option, although the canula line must be properly maintained throughout the retreat by a qualified health professional.⁴
Alternatively, urine sampling is much simpler and more practical than blood sampling and can be performed without medical supervision. Unfortunately, however, since urine osmolality (water content) can vary dramatically depending on the time of day, hydration, and diet, the direct quantification of DMT concentrations in urine cannot provide a reliable measure of its endogenous production and excretion. However, normalisation techniques that provide quantitative drug levels in urine have been established and validated since the 1970s and are included in most guidelines from regulatory bodies such as the Substance Abuse and Mental Health Services Administration (SAMHSA) and the World Anti-Doping Agency (WADA).⁵ The most widely used technique, creatinine normalisation, exploits the fact that creatinine is excreted in the urine at a fairly constant rate. As such, the measured DMT concentration can be normalised by taking its ratio against creatinine concentration, thus providing a quantitative measure of DMT excretion independent of urine osmolality.
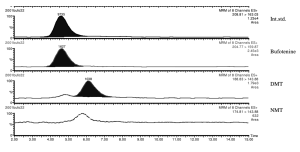
Quantification of DMT, 5-OH-DMT (bufotenine), and NMT in human urine.⁶
The experimental design to test the hypothesis that extended periods of darkness induce endogenous DMT production is thus a relatively simple one: Urine samples are collected at intervals throughout the day and night prior to the darkness retreat to establish baseline levels of DMT and its metabolites in urine. During the darkness retreat itself, urine is collected by the participant at intervals, together with the time the sample is collected, and the collected samples analysed by a third party (using HPLC-MS).⁶ Support for the hypothesis would be obtained if the normalised DMT levels in urine are shown to increase over time during the darkness retreat, relative to baseline levels and using standard tests for statistical significance. Such a result would represent a major breakthrough in our understanding endogenous DMT’s regulation in humans and merit publication in a top tier academic journal.
References
-
Dean, J. G., Liu, T., Huff, S., Sheler, B., Barker, S. A., Strassman, R. J., Wang, M. M., & Borjigin, J. (2019). Biosynthesis and Extracellular Concentrations of N,N-dimethyltryptamine (DMT) in Mammalian Brain. Scientific Reports, 9(1), 9333. https://doi.org/10.1038/s41598-019-45812-w
-
Barker, S. A., McIlhenny, E. H., & Strassman, R. (2012). A critical review of reports of endogenous psychedelic N,N-dimethyltryptamines in humans: 1955-2010. Drug Testing and Analysis, 4(7-8), 617–635. https://doi.org/10.1002/dta.422
-
Callaway, J. C. (1988). A proposed mechanism for the visions of dream sleep. Medical Hypotheses, 26(2), 119–124. https://doi.org/10.1016/0306-9877(88)90064-3
-
Lesser, F. D., Lanham, D. A., & Davis, D. (2020). Blood sampled from existing peripheral IV cannulae yields results equivalent to venepuncture: a systematic review. JRSM Open, 11(5), 2054270419894817. https://doi.org/10.1177/2054270419894817
-
Aydoğdu, M., Oral, S., & Akgür, S. A. (2021). The impact of creatinine reference value: Normalization of urinary drug concentrations. Journal of Forensic Sciences, 66(5), 1855–1861. https://doi.org/10.1111/1556-4029.14739
Cone, E. J., Caplan, Y. H., Moser, F., Robert, T., Shelby, M. K., & Black, D. L. (2009). Normalization of urinary drug concentrations with specific gravity and creatinine. Journal of Analytical Toxicology, 33(1), 1–7. https://doi.org/10.1093/jat/33.1.1
-
Forsström, T., Tuominen, J., & Karkkäinen, J. (2001). Determination of potentially hallucinogenic N-dimethylated indoleamines in human urine by HPLC/ESI-MS-MS. Scandinavian Journal of Clinical and Laboratory Investigation, 61(7), 547–556. https://doi.org/10.1080/003655101753218319

